Okay, I just read another article (from a cannabis science site, no less) that completely missed how flower vaporization actually works. So, I wrote an article for Medium, and wanted to post it on FC for your comments and insights. This was written at a high level for a less knowledgeable audience than y'all. My apologies if this is too basic or has been covered at length elsewhere...
You have surely seen the multi-colored charts that list boiling points of several terpenes and cannabinoids. This information can be helpful, but it’s also quite misleading.
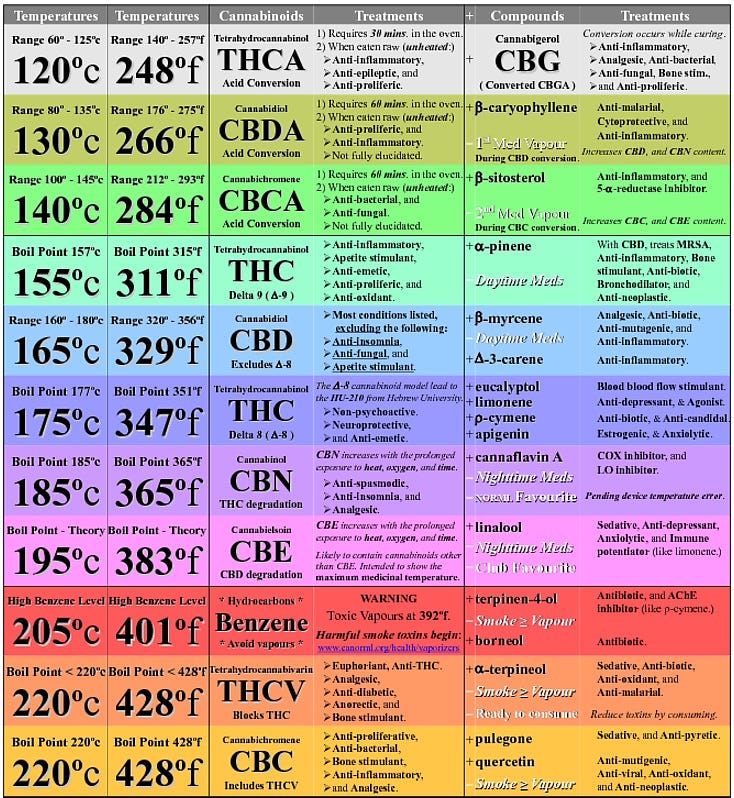
Sample boiling point chart from Reddit
Many of the terpenes found in cannabis are highly volatile and delicate; meaning, they evaporate easily and can be chemically altered or destroyed by high temperatures. There are many misconceptions when it comes to the significance of boiling point temperatures. There is a persistent myth that if you set your vaporizer to a specific temperature, you can dial in a specific terpene or cannabinoid (side note: most vaporizers can NOT actually control to a specific temperature). Similarly, I’ve read articles that imply that if the temperature is below a particular boiling point, then you don’t get much of that compound. And, conversely, if you set the temperature well above the boiling point, you will get all of that compound. Instantly.
Since we can enjoy the rich smells of a dank and terpy bud at room temperature, then clearly terpenes are evaporating at lower temperatures—just like perfume does. Vaporization happens across a wide range of temperatures, not just at specific points. In fact, many terpenes become highly volatile above 70°F, which is why cultivators and extractors try to keep temperatures low during curing and processing to avoid terpene and cannabinoid losses.
There are two primary processes by which liquids vaporize:

Example from https://psiberg.com/evaporation-vs-boiling/
In actuality, evaporation depends heavily on the exposed surface area as well as the temperature and speed of the airflow across that surface. If you’ve ever hung a wet towel out to dry in the sun on a windy day at the beach, you know that evaporation can happen quite quickly. All of the little fiber loops of a terrycloth towel easily wick the moisture and dramatically increase the exposed surface area.
Boiling happens when the temperature of a volume of fluid increases to the point that the microscopic motion of the molecules exert a pressure equal to the pressure of the surrounding atmosphere. Then, vapor forms into bubbles within the fluid, which rise to the surface and burst, releasing the vapor. And, even though boiling is described as a “quick process,” the fluid doesn’t all convert to vapor immediately. It takes time.
So, how does all of this relate to cannabis vaporization? Well, that depends on whether you’re vaporizing flower, extracts/dabs, or cartridges. The same physical principles apply, but the mechanics are quite different.
Vaporizing dried cannabis flower is more similar to the wet towel analogy than to the pot of boiling water. All of the small plant cellulose fibers form an effective wicking matrix for the essential oils and cannabinoids to travel along. When hot air or hot chamber walls come into contact with the trichomes, those melt and a mixture of different plant oils and lipids spread along the fibers, increasing the exposed surface area of the fluid — whereupon they readily evaporate. Except at a very small scale, there just isn’t a volume of liquid for significant boiling to occur.
Thus, rapid evaporation is a more accurate and useful way to think about dried herb vaporization than boiling. However, that is not true for a vape cart or a dab.
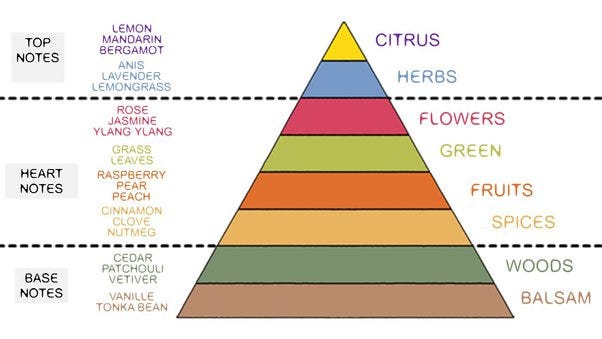
Image source: Olfactory Pyramid or Perfumer’s Nightmare ~ Columns ~ Fragrantica
Setting your vaporizer at a higher temperature will change the rate of evaporation for each of the compounds in the mixture, but it doesn’t mean that you are going to get more of a particular terpene — at least not in a simplistic way. However, the temperature setting is useful in the sense that you can alter the overall blend of terps and cannabinoids you experience with each of your puffs. Just know that subsequent inhalations will contain a different mixture of compounds.
Let me know what you think!
You have surely seen the multi-colored charts that list boiling points of several terpenes and cannabinoids. This information can be helpful, but it’s also quite misleading.

Sample boiling point chart from Reddit
Many of the terpenes found in cannabis are highly volatile and delicate; meaning, they evaporate easily and can be chemically altered or destroyed by high temperatures. There are many misconceptions when it comes to the significance of boiling point temperatures. There is a persistent myth that if you set your vaporizer to a specific temperature, you can dial in a specific terpene or cannabinoid (side note: most vaporizers can NOT actually control to a specific temperature). Similarly, I’ve read articles that imply that if the temperature is below a particular boiling point, then you don’t get much of that compound. And, conversely, if you set the temperature well above the boiling point, you will get all of that compound. Instantly.
Since we can enjoy the rich smells of a dank and terpy bud at room temperature, then clearly terpenes are evaporating at lower temperatures—just like perfume does. Vaporization happens across a wide range of temperatures, not just at specific points. In fact, many terpenes become highly volatile above 70°F, which is why cultivators and extractors try to keep temperatures low during curing and processing to avoid terpene and cannabinoid losses.
What is vaporization, anyway?
Vaporization is a complex process that depends on many different factors including temperature, pressure, surface area, airflow, molecular mass, intermolecular forces, and other effects. In addition, the physical mechanics of the heating device you are using (e.g. convection, conduction, or radiant), can lead to different, and often uneven, vaporization effects. At best, vaporization is poorly understood and often oversimplified.There are two primary processes by which liquids vaporize:
- Evaporation
- Boiling

Example from https://psiberg.com/evaporation-vs-boiling/
In actuality, evaporation depends heavily on the exposed surface area as well as the temperature and speed of the airflow across that surface. If you’ve ever hung a wet towel out to dry in the sun on a windy day at the beach, you know that evaporation can happen quite quickly. All of the little fiber loops of a terrycloth towel easily wick the moisture and dramatically increase the exposed surface area.
Boiling happens when the temperature of a volume of fluid increases to the point that the microscopic motion of the molecules exert a pressure equal to the pressure of the surrounding atmosphere. Then, vapor forms into bubbles within the fluid, which rise to the surface and burst, releasing the vapor. And, even though boiling is described as a “quick process,” the fluid doesn’t all convert to vapor immediately. It takes time.
So, how does all of this relate to cannabis vaporization? Well, that depends on whether you’re vaporizing flower, extracts/dabs, or cartridges. The same physical principles apply, but the mechanics are quite different.
Vaporizing dried cannabis flower is more similar to the wet towel analogy than to the pot of boiling water. All of the small plant cellulose fibers form an effective wicking matrix for the essential oils and cannabinoids to travel along. When hot air or hot chamber walls come into contact with the trichomes, those melt and a mixture of different plant oils and lipids spread along the fibers, increasing the exposed surface area of the fluid — whereupon they readily evaporate. Except at a very small scale, there just isn’t a volume of liquid for significant boiling to occur.
Thus, rapid evaporation is a more accurate and useful way to think about dried herb vaporization than boiling. However, that is not true for a vape cart or a dab.
A complex bouquet of terps
To complicate matters a bit, we aren’t talking about vaporizing just one, homogenous compound. There is a complex bouquet of terpenes, cannabinoids, and flavonoids that get vaporized. And, this bouquet changes over time depending on the relative percentages of the terpenes and how much of each has already been volatilized. In this way, it’s quite similar to how the smell of a perfume evolves on your skin. The lighter “top notes” will evaporate more rapidly than the heavier “base notes,” and the smell of the perfume will evolve during the day.
Image source: Olfactory Pyramid or Perfumer’s Nightmare ~ Columns ~ Fragrantica
Convection or conduction?
Using a pure convection-based vaporizer is the most effective way of experiencing the subtleties and complexities of a finely cultivated, terp-rich strain. These devices deliver heated air that passes through the herb and evaporates the desired compounds. Unfortunately, most vaporizers are not "pure" convection. Conduction-based vaporizers generally involve hot metal or ceramic “ovens” that can overheat the herb where it touches the walls. This intense, localized heat can alter the delicate compounds and lead to that unappealing, “burnt popcorn” flavor. These localized effects are much, much worse in carts where hot coils can directly come in contact with the oils, creating byproducts that have been shown to be toxic.Setting your vaporizer at a higher temperature will change the rate of evaporation for each of the compounds in the mixture, but it doesn’t mean that you are going to get more of a particular terpene — at least not in a simplistic way. However, the temperature setting is useful in the sense that you can alter the overall blend of terps and cannabinoids you experience with each of your puffs. Just know that subsequent inhalations will contain a different mixture of compounds.
Chasing the taste
So, if you want to savor more of those lighter, delicate notes, set your vaporizer as low as possible. You’ll still get those heavier terpenes as well, but in a smaller percentage early on in your bowl. If you desire a denser vapor, you can set it higher to introduce some of those deeper, nuttier tastes into the vapor early. And, higher temperatures also alter the rate at which the flower undergoes decarboxylation…which, is NOT instantaneous when vaporizing. But, that is a topic for another day.Let me know what you think!
Last edited:



