herbivore21
Well-Known Member
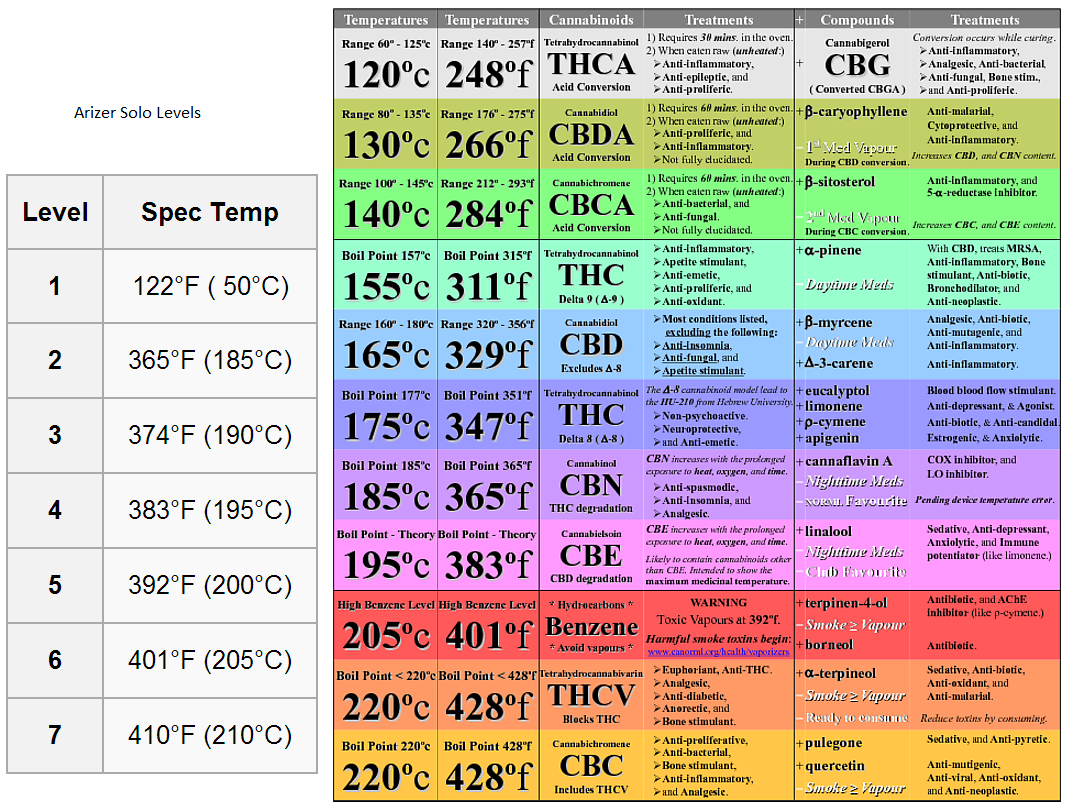
Thanks for this brother, you totally saved me from having to explain this again (I swear I am explaining this point every other day lol).Yes, pure samples of Benzene when heated in a vacuum have a boiling point of 392 F or so.
But, we don't vape pure isolates of any of the products in MJ and what products we do extract are from a complex plant structure so the prevailing thought is that this smears out the boiling point into a range at which you may see this product.
I also note that in the tables on this subject that I have seen, the % of benzene is always a question mark. Not sure why but I presume that if it was released in any significant qty that it would be detected and listed, no?
We might also add that even where studies have found that cannabis flower releases benzene in some detectable amount (remember benzene limits from the OSHA are in the single-double digits of ppm IIRC), cannabis flower contains a lot of inactive plant components that are prone to substantial thermal-oxidative decomposition long before the essential oil fraction starts to degrade. Take that plant material out, and you may not have any benzene produced at all from a concentrate. You also might find that even if you can still produce harmful byproducts from essential oil preparations, that the temp to cause such decomposition will be considerably higher than a dry, ground herb because we are dealing with a viscous liquid instead whose content is quite different.
Last edited: