tepictoton
Well-Known Member
http://www.fundacion-canna.es/en/vaporizer-usage-safety-and-toxicology
Vaporizer Usage: safety and toxicology
Whether by medical recommendation or by one’s own decision, the consumption of any substance always entails a series of risks, speaking in terms of the health of the organism. Those risks not only form part of the specific properties of the consumed substance itself; the method of consumption also has a series of implications for our bodies (e.g. eatables could last too long or give unpredictable effects). In this article, we center on safety and toxicity, when using a vaporizer, comparing it with traditional and more common usage by combustion, whilst also considering certain technical aspects, with regard to design, materials and certifications. Our objective is to achieve some guarantees for usage close to its medical or therapeutic application.
Aida Acero y Jorge Fernández
Combustion
With regard to the combustion of the cannabis plant, it hardly needs saying that most of the toxicity is in the smoke. Although certain components are absent that are found in tobacco, the smoke arising from the combustion of any plant contains tar, certain polynuclear hydrocarbons that are potentially carcinogenic and other harmful compounds. Neither can we, of course, forget carbon monoxide.
Method Vaporization Combustion
Temperature Up to 230ºC 230º – 900ºC
Extraction of Active principles
Aroma, flavor…
Other substances Active principles
Aroma, flavor,…
Other substances
Destruction of Does not destroy active principles.
An excess of heat by conduction
destroys terpenes Active Principles by burning
Formation of Activates the bioavailability of certain active principles by temperature (decarboxylation)
Traces of CO2 and pyrolysis at over 230º C
A recent article warms for formation of ammonia from vaporizing Activates the bioavailability of certain active principles by temperature
CO2 + CO
Tar (polycyclic aromatic hydrocarbons)
Other harmful compounds
(toluene, naphthalene, benzene, nitrosamines, hydrogen cyanide, ammonium hydride,…)
Table 1. Summary of the differences between vaporization and combustion
When smoking, we might add that not only are we talking about compounds in a gaseous state but also of small-sized solid particles that adhere to the lungs, which after prolonged use finally reduce our pulmonary capacity or become precursors of diseases that principally affect the respiratory tract (throat, trachea, lungs…)
Looking more closely at the combustion methods, it would be necessary to differentiate between the hand-rolled spliff or joint, and other utensils such as pipes, hookahs, and various water-based tools (e.g. water pipe). Beginning with the use of this last group, we reduce the quantity of solid particles by filtering with water or condensate, and we avoid the toxins coming from the slow-combustion of paper. It is true that there are more organic papers, the manufacture of which by-passes certain chemical processes, but it has not been demonstrated that their use avoids the formation of toxic substances. Anyway the largest proportion of toxins is produced by the combustion of the plant itself.
With reference to the materials from which smoking utensils are made, the principle concerns are the small particles that they can give off and the durability of those materials against daily use and high temperatures. There are materials of proven purity like borosilicate glass; but other materials such as copper and certain nickel-based alloys, are an example of how high temperatures can transform them into inhalable oxides with demonstrated carcinogenic effects.
Vaporization
With regard to vaporization, if the basic goal is to raise the temperature of a substance to such a point that you manage to extract its active principles without going so far as to produce combustion; it implies that no smoke is obtained in inhalation and therefore no toxic components are present in solid or gaseous forms.
However, it should be taken into account that some vaporizers can exceed the temperature of pyrolysis (spontaneous combustion) of the cellulose when the plant is heated up above 230º C.
This generates similar residues to those from combustion, although in the majority of cases the quantity of toxins is much smaller than smoking [Gieringer, 1996; McPartland, 1997].
Nonfilter Cigarette Filter Cigarette Waterpipe #1 Waterpipe #2 Vaporizer #1 Vaporizer #2
Total Tars (mg/puff) 309.8 140.5 24.5 9.2 4.76 11.3
Total Cannabinoids (% Tar) 7.82 5.32 5.46 4.48 7.89 9.82
TABLE 1. Tar and Cannabinoid Delivery–7 Smoking Devices
Adapted from Gieringer, D. “Marijuana Waterpipe and Vaporizer Study,” 1996
The materials of the vaporizer itself can also be harmful to the health of users. Those materials used for the manufacture of a vaporizer should be resistant to high temperatures as well as clean and long-lasting. With the aim of testing the safety of the materials that are used, it is important that the manufacturer test the device through various laboratory procedures: on the one hand, the degradation/formation of gaseous and potentially toxic substances for the user, but on the other, the release of solid compounds that can accumulate in the body.
Each vaporizer manufacturer should order the preparation of a toxicology report, detailing the methods and tests to guarantee that the device is safe and innocuous. All materials that have any contact with the flow of air/vapor from the device or from the substance should be tested, because their degradation by temperature or its use under extreme conditions could imply a risk when inhaled by the user. The objective is to test whether there are any detached materials and that they are below legal safely limits (metals such as: aluminum, chrome, manganese, magnesium, copper, silicon, zinc, iron; and whether toxic substances exist in the form of vapor, which in the case of plastics may be poly-fluorinated compounds (PFCs) and per-fluorinated alkaline phosphates (PAPs), for example.
The condensed air from the empty (with no load of vegetable material) vaporizer should also be analyzed by conducting simulated aspirations in frequency/time of use. For example: to simulate the case of an individual who uses the vaporizer 6 times a day for 2 years under the highest conditions for temperature and inhalation intensity.
Testing the device
The PEL (Permissible Exposure Limits) standards specified by the Occupational Safety and Health Administration (OSHA) of the United States are used to arrive at a real comparison. In addition, scientific publications must be reviewed in such fields as: the deterioration of plastic materials because of the effect of temperature; the chemical composition of metallic alloys and their behavior against thermal stress; and their exposure to corrosive agents, among others.
In other cases, simple observation of materials that form the air duct very often indicate whether there are particles that have detached or that are not described by the manufacturer.
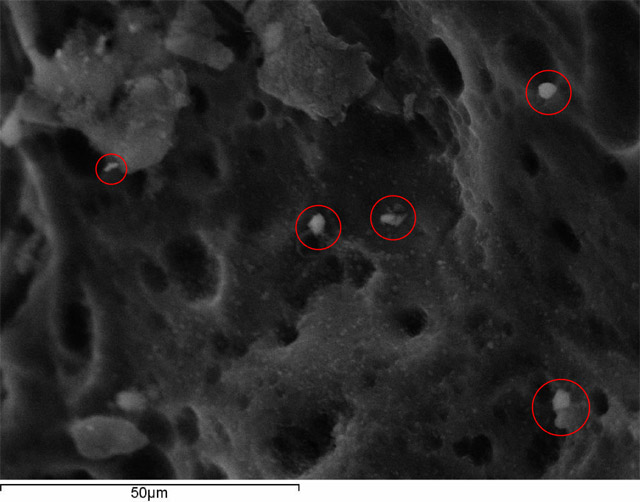
Figure 2. SEM (Scanning Electron Microscopy) analysis of the heat exchanger of the miniVAP. Dust particles from the process of both handling and manufacturing are visible before its cleaning.
Other factors
There are other aspects on safety in vaporization that correspond to a series of standards or norms. For example, those related to electricity safety, although it is also interesting to take into account the policy of the manufacturer and its consequences for the environment: programmed obsolescence, choice of raw materials, waste recycling, etc.
Vaporizer Usage: safety and toxicology
Whether by medical recommendation or by one’s own decision, the consumption of any substance always entails a series of risks, speaking in terms of the health of the organism. Those risks not only form part of the specific properties of the consumed substance itself; the method of consumption also has a series of implications for our bodies (e.g. eatables could last too long or give unpredictable effects). In this article, we center on safety and toxicity, when using a vaporizer, comparing it with traditional and more common usage by combustion, whilst also considering certain technical aspects, with regard to design, materials and certifications. Our objective is to achieve some guarantees for usage close to its medical or therapeutic application.
Aida Acero y Jorge Fernández
Combustion
With regard to the combustion of the cannabis plant, it hardly needs saying that most of the toxicity is in the smoke. Although certain components are absent that are found in tobacco, the smoke arising from the combustion of any plant contains tar, certain polynuclear hydrocarbons that are potentially carcinogenic and other harmful compounds. Neither can we, of course, forget carbon monoxide.
Method Vaporization Combustion
Temperature Up to 230ºC 230º – 900ºC
Extraction of Active principles
Aroma, flavor…
Other substances Active principles
Aroma, flavor,…
Other substances
Destruction of Does not destroy active principles.
An excess of heat by conduction
destroys terpenes Active Principles by burning
Formation of Activates the bioavailability of certain active principles by temperature (decarboxylation)
Traces of CO2 and pyrolysis at over 230º C
A recent article warms for formation of ammonia from vaporizing Activates the bioavailability of certain active principles by temperature
CO2 + CO
Tar (polycyclic aromatic hydrocarbons)
Other harmful compounds
(toluene, naphthalene, benzene, nitrosamines, hydrogen cyanide, ammonium hydride,…)
Table 1. Summary of the differences between vaporization and combustion
When smoking, we might add that not only are we talking about compounds in a gaseous state but also of small-sized solid particles that adhere to the lungs, which after prolonged use finally reduce our pulmonary capacity or become precursors of diseases that principally affect the respiratory tract (throat, trachea, lungs…)
Looking more closely at the combustion methods, it would be necessary to differentiate between the hand-rolled spliff or joint, and other utensils such as pipes, hookahs, and various water-based tools (e.g. water pipe). Beginning with the use of this last group, we reduce the quantity of solid particles by filtering with water or condensate, and we avoid the toxins coming from the slow-combustion of paper. It is true that there are more organic papers, the manufacture of which by-passes certain chemical processes, but it has not been demonstrated that their use avoids the formation of toxic substances. Anyway the largest proportion of toxins is produced by the combustion of the plant itself.
With reference to the materials from which smoking utensils are made, the principle concerns are the small particles that they can give off and the durability of those materials against daily use and high temperatures. There are materials of proven purity like borosilicate glass; but other materials such as copper and certain nickel-based alloys, are an example of how high temperatures can transform them into inhalable oxides with demonstrated carcinogenic effects.
Vaporization
With regard to vaporization, if the basic goal is to raise the temperature of a substance to such a point that you manage to extract its active principles without going so far as to produce combustion; it implies that no smoke is obtained in inhalation and therefore no toxic components are present in solid or gaseous forms.
However, it should be taken into account that some vaporizers can exceed the temperature of pyrolysis (spontaneous combustion) of the cellulose when the plant is heated up above 230º C.
This generates similar residues to those from combustion, although in the majority of cases the quantity of toxins is much smaller than smoking [Gieringer, 1996; McPartland, 1997].
Nonfilter Cigarette Filter Cigarette Waterpipe #1 Waterpipe #2 Vaporizer #1 Vaporizer #2
Total Tars (mg/puff) 309.8 140.5 24.5 9.2 4.76 11.3
Total Cannabinoids (% Tar) 7.82 5.32 5.46 4.48 7.89 9.82
TABLE 1. Tar and Cannabinoid Delivery–7 Smoking Devices
Adapted from Gieringer, D. “Marijuana Waterpipe and Vaporizer Study,” 1996
The materials of the vaporizer itself can also be harmful to the health of users. Those materials used for the manufacture of a vaporizer should be resistant to high temperatures as well as clean and long-lasting. With the aim of testing the safety of the materials that are used, it is important that the manufacturer test the device through various laboratory procedures: on the one hand, the degradation/formation of gaseous and potentially toxic substances for the user, but on the other, the release of solid compounds that can accumulate in the body.
Each vaporizer manufacturer should order the preparation of a toxicology report, detailing the methods and tests to guarantee that the device is safe and innocuous. All materials that have any contact with the flow of air/vapor from the device or from the substance should be tested, because their degradation by temperature or its use under extreme conditions could imply a risk when inhaled by the user. The objective is to test whether there are any detached materials and that they are below legal safely limits (metals such as: aluminum, chrome, manganese, magnesium, copper, silicon, zinc, iron; and whether toxic substances exist in the form of vapor, which in the case of plastics may be poly-fluorinated compounds (PFCs) and per-fluorinated alkaline phosphates (PAPs), for example.
The condensed air from the empty (with no load of vegetable material) vaporizer should also be analyzed by conducting simulated aspirations in frequency/time of use. For example: to simulate the case of an individual who uses the vaporizer 6 times a day for 2 years under the highest conditions for temperature and inhalation intensity.
Testing the device
The PEL (Permissible Exposure Limits) standards specified by the Occupational Safety and Health Administration (OSHA) of the United States are used to arrive at a real comparison. In addition, scientific publications must be reviewed in such fields as: the deterioration of plastic materials because of the effect of temperature; the chemical composition of metallic alloys and their behavior against thermal stress; and their exposure to corrosive agents, among others.
In other cases, simple observation of materials that form the air duct very often indicate whether there are particles that have detached or that are not described by the manufacturer.

Figure 2. SEM (Scanning Electron Microscopy) analysis of the heat exchanger of the miniVAP. Dust particles from the process of both handling and manufacturing are visible before its cleaning.
Other factors
There are other aspects on safety in vaporization that correspond to a series of standards or norms. For example, those related to electricity safety, although it is also interesting to take into account the policy of the manufacturer and its consequences for the environment: programmed obsolescence, choice of raw materials, waste recycling, etc.