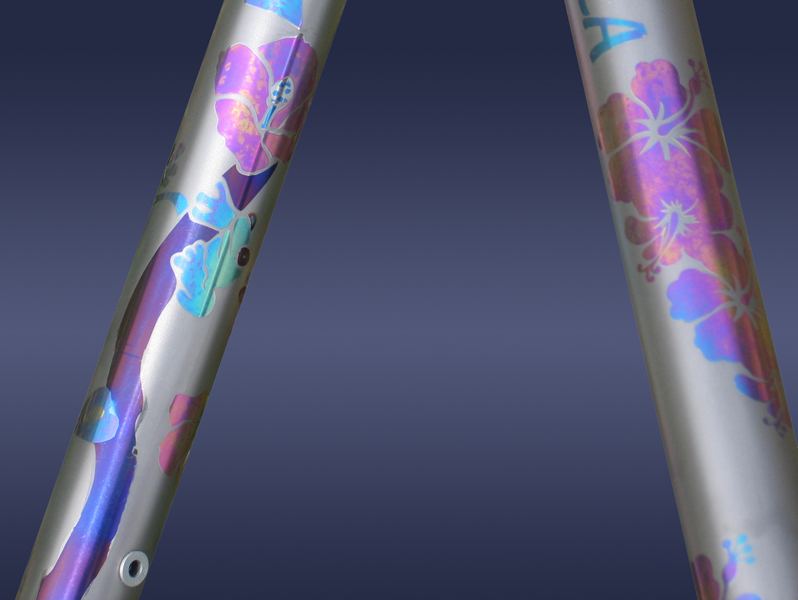
And at this pic is the full range of hues that can Titanium can get .
The chart is about another reactive metal ( NIOBIUM ,Nb ) ,
but it applies exactly as is for Titanium as well.
Notice that true pink is achieved at 125 to 140 VDC.
Way to high voltage.
Usually anodising Titanium to achieve hues higher than second order blue ( > 80 VDC ) ,
is very tricky and involves nasty chemicals for etching titanium surface,prior to anodising.
Still some just get lucky enough and actually manage to get those hues ,
without etching.
Pink though (125-140 VDC ) is out of question,without etching Ti with HF
( Hydrofluoric acid ,can kill or cause severe injuries ,very easily .Even breathing it's fumes
can be fatal. )
HF aka Hydrofluoric acid
" Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. It is a precursor to almost all fluorine compounds, including pharmaceuticals such as fluoxetine (Prozac), diverse materials such as PTFE (Teflon), and elemental fluorine itself. It is a colourless solution that is highly corrosive, capable of dissolving many materials, especially oxides.
Its ability to dissolve glass has been known since the 17th century, even before Carl Wilhelm Scheele prepared it in large quantities in 1771.[2] Because of its high reactivity toward glass and moderate reactivity toward many metals, hydrofluoric acid is usually stored in plastic containers (although PTFE is slightly permeable to it).[3]
Hydrogen fluoride gas is an acute poison that may immediately and permanently damage lungs and the corneas of the eyes. Aqueous hydrofluoric acid is a contact-poison with the potential for deep, initially painless burns and ensuing tissue death. By interfering with body calcium metabolism, the concentrated acid may also cause systemic toxicity and eventual cardiac arrest and fatality, after contact with as little as 160 cm2 (25 square inches) of skin. "
" In addition to being a highly corrosive liquid, hydrofluoric acid is also a powerful contact poison. Because of the ability of hydrofluoric acid to penetrate tissue, poisoning can occur readily through exposure of skin or eyes, or when inhaled or swallowed. Symptoms of exposure to hydrofluoric acid may not be immediately evident, and this can provide false reassurance to victims, causing them to delay medical treatment.[9] Despite having an irritating odor, HF may reach dangerous levels without an obvious odor.[5] HF interferes with nerve function, meaning that burns may not initially be painful. Accidental exposures can go unnoticed, delaying treatment and increasing the extent and seriousness of the injury.[9] Symptoms of HF exposure include irritation of the eyes, skin, nose, and throat, eye and skin burns, rhinitis, bronchitis, pulmonary edema (fluid buildup in the lungs), and bone damage.[10]
Once absorbed into blood through the skin, it reacts with blood calcium and may cause cardiac arrest. Burns with areas larger than 160 cm2 (25 square inches) have the potential to cause serious systemic toxicity from interference with blood and tissue calcium levels.[11] In the body, hydrofluoric acid reacts with the ubiquitous biologically important ions Ca2+ and Mg2+. Formation of insoluble calcium fluoride is proposed as the etiology for both precipitous fall in serum calcium and the severe pain associated with tissue toxicity.[12] In some cases, exposures can lead to hypocalcemia. Thus, hydrofluoric acid exposure is often treated with calcium gluconate, a source of Ca2+ that sequesters the fluoride ions. HF chemical burns can be treated with a water wash and 2.5% calcium gluconate gel[13][14][15] or special rinsing solutions.[16][17] However, because it is absorbed, medical treatment is necessary;[11] rinsing off is not enough. Intra-arterial infusions of calcium chloride have also shown great effectiveness in treating burns.[18]
Hydrogen fluoride is generated upon combustion of many fluorine-containing compounds such as products containing Viton and polytetrafluoroethylene (Teflon) parts.[19] Hydrofluorocarbons in automatic fire suppression systems can release hydrogen fluoride at high temperatures, and this has led to deaths from acute respiratory failure in military personnel when a rocket-propelled grenade hit the fire suppression system in their vehicle.[20] Hydrofluoric acid can be released from volcanoes, sea salt aerosol, and from welding or manufacturing processes. "
https://en.wikipedia.org/wiki/Hydrofluoric_acid
Careful with the Viton O-rings.
If one burns accidently (I'm not sure how ,though ) his /her Viton O-rings of the tip,
nasty HF fumes will be released .


























 , Sailor!
, Sailor!