Nebulous
Active Member
Hey all, I've got a chemistry-based question about what our favorite activity should be called from a scientific standpoint.
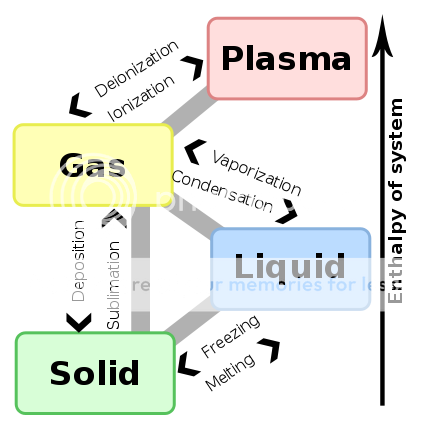
In chemistry, when a substance changes from gas to liquid, liquid to solid, etc, the process is called a phase transition.
http://en.wikipedia.org/wiki/Phase_transition
Vaporization is defined in chemistry as the change from liquid to gas state. When you boil water, you are "vaporizing" the liquid water molecules into gaseous water molecules.
My question is if the psycho-active compounds in marijuana are vaporized from a liquid to gaseous state. As it appears the compounds in our weed are initially in a solid state (we dry the stuff after all), the act of chemical "vaporization" would subsequently require melting of the compounds from a solid to liquid state, and then boiling/vaporizing them into gaseous vapor state. I don't think this is the case. Instead, I believe our vaporizers achieve the act of sublimation, which is direct transition from solid to gas, bypassing the liquid state. A classic example of sublimation is dry ice - the solid ice cubes turn to gas without melting.
What a chemist would call our notion of vaporization may be largely irrelevant to the task at hand, but I find it interesting to think that I am actually subliming my herb instead of vaporizing. And of course, sublimation calls to mind sublime, which is a word we can all agree describes the vaporization experience quite well
This diagram is useful when trying to visualize the various phase transitions.
In chemistry, when a substance changes from gas to liquid, liquid to solid, etc, the process is called a phase transition.
http://en.wikipedia.org/wiki/Phase_transition
Vaporization is defined in chemistry as the change from liquid to gas state. When you boil water, you are "vaporizing" the liquid water molecules into gaseous water molecules.
My question is if the psycho-active compounds in marijuana are vaporized from a liquid to gaseous state. As it appears the compounds in our weed are initially in a solid state (we dry the stuff after all), the act of chemical "vaporization" would subsequently require melting of the compounds from a solid to liquid state, and then boiling/vaporizing them into gaseous vapor state. I don't think this is the case. Instead, I believe our vaporizers achieve the act of sublimation, which is direct transition from solid to gas, bypassing the liquid state. A classic example of sublimation is dry ice - the solid ice cubes turn to gas without melting.
What a chemist would call our notion of vaporization may be largely irrelevant to the task at hand, but I find it interesting to think that I am actually subliming my herb instead of vaporizing. And of course, sublimation calls to mind sublime, which is a word we can all agree describes the vaporization experience quite well

This diagram is useful when trying to visualize the various phase transitions.



 ) but I sure am interested int this.
) but I sure am interested int this.