started@52
Well-Known Member
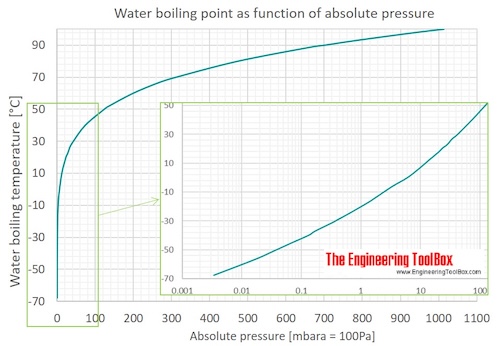
I live around 5500 feet above sea level and wonder if that’s why I seam to generally vape and get clouds at lower temps then many others. Water boils about 9-10° lower here around 202-203° So I’m assuming other things vape at lower temps too?
I will be going down to about 3000’ soon and doubt I’ll notice a big difference, but what if I vaped at sea level? I haven’t been vaping very long and have not vaped much if at all at higher or lower altitudes.
I will be going down to about 3000’ soon and doubt I’ll notice a big difference, but what if I vaped at sea level? I haven’t been vaping very long and have not vaped much if at all at higher or lower altitudes.